BASIC STRUCTURE OF MATTER
After reading this section you will be able to do the following:
- Give a basic definition of matter.
- Describe a molecule.
The Atom
All matter such as solids, liquids, and gases, is composed of atoms. Therefore, the atom is considered to be the basic building block of matter. However, atoms are almost always grouped together with other atoms to form what is called a molecule. Only a few gases such as helium are composed of individual atoms as the structural unit.
Atoms are extremely small. The radius of a typical atom is on the order of 0.00000000001 meter and cannot be studied without very powerful microscopes. In the image below you can see how a scanning electron microscope can be used to magnify things until very small details appear relatively big.
In the next two sub-units you will learn about what a material composed of only one type of atom is called and you will look at the basic model of an atom.
Review
- All matter is composed of atoms.
- A molecule is a group of atoms bunched together.
ELEMENTS
After reading this section you will be able to do the following:
- Be able to define an element.
- Be able to recognize and recall some of the basic elements.
An element defined
Any material that is composed of only one type of atom is called a chemical element, a basic element, or just an element. Any material that is composed of more than one type of atom is called a compound. Every element has a unique atomic structure. Scientists know of only about 109 basic elements at this time. (This number has a habit of changing.) All matter is composed of combinations of one or more of these elements. Ninety-one of these basic elements occur naturally on or in the Earth. The other elements are man-made. You may recognize the names of some of these basic elements, such as: hydrogen, helium, oxygen, iron, copper, gold, aluminum, uranium. The periodic table of elements (shown below) lists the basic elements and some of their properties.
Review
- An element is material composed of only one kind of atom.
- A compound is material composed of more than one kind of atom.
- Some examples of elements that can be found on the periodic table are hydrogen, helium, oxygen, iron, copper, gold, aluminum, uranium.
ATOM MODELS
After reading this section you will be able to do the following:
- Describe the basic structure of an atom.
- Explain what holds an atom together.
What is an atom composed of?
An atom is the smallest particle of any element that still retains the characteristics of that element. However, atoms consist of even smaller particles. Atoms consist of a central, dense nucleus that is surrounded by one or more lightweight negatively charged particles called electrons. The nucleus is made up of positively charged particles called protons and neutrons which are neutral. An atom is held together by forces of attraction between the electrons and the protons. The neutrons help to hold the protons together. Protons and neutrons are believed to be made up of even smaller particles called quarks. We will limit our discussions to protons, neutrons and electrons.

Niels Bohr was a Danish scientist who introduced the model of an atom in 1913. Bohr's model consists of a central nucleus surrounded by tiny particles called electrons that are orbiting the nucleus in a cloud. These electrons are spinning so fast around the nucleus of the atom that they would be just a blur if we could see particles that small. In our pictures and exercises the electron appears to orbit in the same path around the nucleus much like the planets orbit the Sun. But, please be aware that electrons do not really orbit in the same path. The electrons actually change their orbit with each revolution.
Review
- Atoms are composed of protons, neutrons, and electrons.
- The forces of attraction between the electrons and the protons hold an atom together.
ELECTRIC CHARGE
After reading this section you will be able to do the following:
- Explain the differences between electrons and protons.
- Predict what happens when protons and electrons interact with other protons or electrons.
Electrons
 Electrons are the smallest and lightest of the particles in an atom. Electrons are in constant motion as they circle around the nucleus of that atom. Electrons are said to have a negative charge, which means that they seem to be surrounded by a kind of invisible force field. This is called anelectrostatic field.
Electrons are the smallest and lightest of the particles in an atom. Electrons are in constant motion as they circle around the nucleus of that atom. Electrons are said to have a negative charge, which means that they seem to be surrounded by a kind of invisible force field. This is called anelectrostatic field.Protons
 Protons are much larger and heavier than electrons. Protons have a positive electrical charge. This positively charged electrostatic field is exactly the same strength as the electrostatic field in an electron, but it is opposite in polarity. Notice the negative electron (pictured at the top left) and the positive proton (pictured at the right) have the same number of force field lines in each of the diagrams. In other words, the proton is exactly as positive as the electron is negative.
Protons are much larger and heavier than electrons. Protons have a positive electrical charge. This positively charged electrostatic field is exactly the same strength as the electrostatic field in an electron, but it is opposite in polarity. Notice the negative electron (pictured at the top left) and the positive proton (pictured at the right) have the same number of force field lines in each of the diagrams. In other words, the proton is exactly as positive as the electron is negative.
Like charges repel, unlike charges attract
Two electrons will tend to repel each other because both have a negative electrical charge. Two protons will also tend to repel each other because they both have a positive charge. On the other hand, electrons and protons will be attracted to each other because of their unlike charges.
Since the electron is much smaller and lighter than a proton, when they are attracted to each other due to their unlike charges, the electron usually does most of the moving. This is because the protons have more mass and are harder to get moving. Although electrons are very small, their negative electrical charges are still quite strong. Remember, the negative charge of an electron is the same as the positive electrical charge of the much larger in size proton. This way the atom stays electrically balanced.
Another important fact about the electrical charges of protons and electrons is that the farther away they are from each other, the less force their electric fields have on each other. Similarly, the closer they are to each other, the more force they will experience from each other due to this invisible force field called an electric field.
Review
- Electrons have a negative electrostatic charge and protons have a positive electrostatic charge.
- A good way to remember what charge protons have is to remember both proton and positive charge start with "P."
- Like charges repel, unlike charges attract, just like with magnets.
THE FREE ELECTRON
After reading this section you will be able to do the following:
- Explain how electrons are arranged in an atom.
- Describe how elements maintain their electrical balance.
Maintaining electrical balance
Each basic element has a certain number of electrons and protons, which distinguishes each element from all other basic elements. In most elements, the number of electrons is equal to the number of protons. This maintains an electrical balance in the structure of atoms since protons and electrons have equal, but opposite electrostatic fields.

Pictured here is an atom of copper, which is much more complex than either an atom of hydrogen or helium.
The copper atom has 29 protons in its nucleus with 29 electrons orbiting the nucleus. Notice that in the copper atom, the electrons are arranged in several layers called shells. This is to graphically represent that the electrons are at different energy levels within the atom. The energy of an electron is restricted to a few particular energy levels. The energy is said to be quantized, meaning that it cannot vary continuously over a range, but instead is limited to certain values. These energy levels or shells follow a very predictable pattern. The closest shell to the nucleus can have up to 2 electrons. The second shell from the nucleus can have up to 8 electrons. The third shell can have up to 18 electrons. The fourth shell can have up to 32 electrons, and so on. Atoms can have this many electrons, but they do not have to have this many electrons in each shell. The greater distance between the electrons in the outer shells and the protons in the nucleus mean the outer shell electrons experience less of a force of attraction to the nucleus than do the electron in the inner shells.
In the next sub-unit you will learn about the the outer shell of an atom called the valence shell.
Review
- Atoms have their electrons arranged in layers called shells.
- In order to maintain electrical balance the number of electrons is equal to the number of protons in most elements.
THE VALENCE SHELL
After reading this section you will be able to do the following:
- Give an explanation of the valence shell of an atom.
- Explain what free electrons are and why they are important.
What is the valence shell?
Notice that in the copper atom pictured below that the outside shell has only one electron. This represents that the copper atom has one electron that is near the outer portion of the atom. The outer shell of any atom is called the valence shell. When the valence electron in any atom gains sufficient energy from some outside force, it can break away from the parent atom and become what is called a free electron.

Pictured here is an atom of copper, which is much more complex than either an atom of hydrogen or helium.
Atoms with few electrons in their valence shell tend to have more free electrons since these valence electrons are more loosely bound to the nucleus. In some materials like copper, the electrons are so loosely held by the atom and so close to the neighboring atoms that it is difficult to determine which electron belongs to which atom. Under these conditions, the valence or free electrons tend to drift randomly from one atom to its neighboring atoms. Under normal conditions the movement of the electrons is truly random, meaning they are moving in all directions by the same amount. However, if some outside force acts upon the material, this flow of electrons can be directed through materials and this flow is called electrical current. Materials that have free electrons and allow electrical current to flow easily are called conductors. Many materials do not have any free electrons. Because of this fact, they do not tend to share their electrons very easily and do not make good conductors of electrical currents. These materials are called insulators. There will be more information on this later.
Review
- The valence shell is the outer shell of the atom.
- Some materials have a free electron in their valence shell and this electron can easily move from atom to atom.
- The free electrons are responsible for electrical current.
 |  |  |
| Microscopic view of a gas. | Microscopic view of a liquid. | Microscopic view of a solid. |
- Particles in a:
- gas are well separated with no regular arrangement.
- liquid are close together with no regular arrangement.
- solid are tightly packed, usually in a regular pattern.
- Particles in a:
- gas vibrate and move freely at high speeds.
- liquid vibrate, move about, and slide past each other.
- solid vibrate (jiggle) but generally do not move from place to place.
| Some Characteristics of Gases, Liquids and Solids and the Microscopic Explanation for the Behavior | ||
|---|---|---|
| gas | liquid | solid |
| assumes the shape and volume of its container particles can move past one another | assumes the shape of the part of the container which it occupies particles can move/slide past one another | retains a fixed volume and shape rigid - particles locked into place |
| compressible lots of free space between particles | not easily compressible little free space between particles | not easily compressible little free space between particles |
| flows easily particles can move past one another | flows easily particles can move/slide past one another | does not flow easily rigid - particles cannot move/slide past one another |
Particulate nature of matter. Matter is made up of millions of tiny particles which cannot be seen with naked eyes. These particles are called atoms and are made up of sub-atomic particles called protons, neutrons and electrons. Atoms join together to form molecules
BROWNIAN MOTION
The explanation is based on the kinetic theory of matter and justifies the movement of pollen particles (so called brownian particles) by their collisions with much smaller water molecules. ...Brownian motion is considered one of the experimental proofs that particles in matter constantly move in a disordered fashion.
Brownian motion or pedesis is the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving molecules in the fluid.
This is a simulation of the Brownian motion of 5 particles (yellow) that collide with a large set of 800 particles. The yellow particles leave 5 blue trails of random motion and one of them has a red velocity vector.
KINETIC THEORY
Kinetic theory or kinetic theory of gases attempts to explain overall properties of gases, such as pressure, temperature, or volume, by considering their molecular composition and motion. The theory basically states that pressure is not caused by molecules pushing each other away, like earlier scientists thought. Instead, pressure is caused by the molecules colliding with each other and their container. Kinetic theory is also known as kinetic-molecular theory or collision theory.
There are three main components to kinetic theory:
· No energy is gained or lost when molecules collide
· The molecules in a gas take up a negligible (able to be ignored) amount of space in relation to the container they occupy
· The molecules are in constant, linear motion
Kinetic theory of gases explains the macroscopic properties of gases, such as pressure in a gas, evaporation and boiling; cohesion, adhesion, capillarity viscosity, thermal conductivity, and volume, by considering their molecular composition and motion. The theory posits that gas pressure results from particles' collisions with the walls of a container at different velocities.
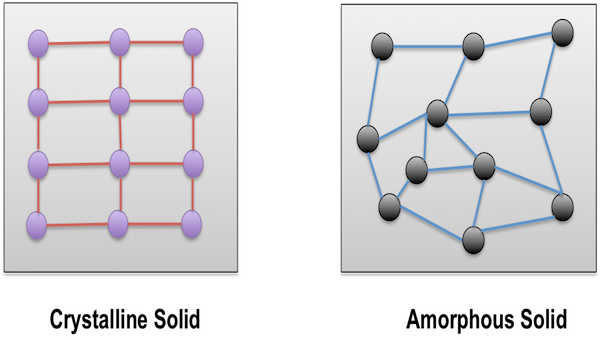
- Amorphous Solids
Image 1: Arrangement of Atoms
Amorphous Solids
The solids in which the constituent particles of matter are arranged in a random manner are called amorphous solids. It is a non-crystalline solid with no proper arrangement of atoms in the solid lattice. In other words, we can define amorphous solids as materials which don’t have certain organized arrangement of atoms and molecules. Most solids are amorphous in nature and are utilized in many sectors as well. One of the most common examples of amorphous solids is glass, which is used widely in the manufacturing sector.
Characteristics of Amorphous Solids
An Amorphous Solid depicts following properties, which are as follows:
- The constituent particles of matter inside solid are arranged in a random manner, that is, the position of atoms and molecules is not fixed and varies from one solid to another
- Amorphous Solids don’t have definite shape or geometry due to random arrangement of atoms and molecules inside the solid lattice
- Short-range order is found in amorphous solids
- Amorphous Solids are also called Pseudo-solids or Supercooled Liquids because they don’t form crystalline structure and has the ability to flow
- The nature of amorphous solids is isotropic in nature that is, the properties measured in all directions come out to be same, example refractive index of amorphous solids is same
- Amorphous solids don’t show sharp melting point, this is because of irregular packing of amorphous solids
- When we cut an amorphous solid, we find the broken constituent particles to be irregular in shape and geometry
- Amorphous solids are unsymmetrical in nature, due to irregular packing of atoms and molecules inside the solid lattice
- Amorphous solids don’t have fixed heat of fusion because of absence of sharp melting point
Examples: Plastics, Glass, Rubber, Metallic Glass, Polymers, Gel etc.
There are many applications of amorphous solids, some of them are:
- The glass is widely used in packaging (food jars, cosmetics box, and soft-drink bottles), making tableware (utensils), in the construction of buildings ( windows, lighting, and shelves) etc.
- Rubber is mainly used in manufacturing of tires, footwear, ropes, camp cloth and as a raw material for several industries
- Use of polymer can be seen in manufacturing of pipes, medicines and as a raw ingredient for many factories
- Amorphous silicon is considered as the best photovoltaic material to convert sunlight into electricity
Crystalline Solids
The solids in which the constituent particles of matter are arranged and organized in a specific manner are called Crystalline Solids. These solids contain crystals in their structure and each crystal has definite geometry. Adding further, as crystalline solids have low potential energy, they are the most stable form of solids. Almost all solids fall in the category of crystalline solids including metallic elements (iron, silver, and copper) and non-metallic elements (Phosphorus, Sulphur, and iodine). Also several compounds like sodium chloride, zinc sulphide and naphthalene build crystalline solids.
Image 2: Example of Crystalline Solids
Characteristics of Crystalline Solids
The main characteristics of crystalline solids are mentioned as below:
- Crystalline solids show regular structure and have definite geometrical shape
- The sharp freezing point is found in crystalline solids. This is because the distance between same atoms/molecules or ions is same and remains constant, unlikely from amorphous solids
- The heat of fusion is definite and fixed as the regularity in crystal lattice remains same and is ideal
- Crystalline Solids are also known as True Solids as they don’t tend to flow like pseudo solids
- When we cut a crystal solids with a knife, we obtain a flat and smooth surface
- The nature of crystalline solid is anisotropic; that is, the properties turn out to be different in different direction
- Crystalline solids depict both long range and short range order
Examples: Quartz, Calcite, Sugar, Mica, Diamonds etc.
Image 3: Lattice Structure of Crystalline Solids
Uses of Crystalline Solids
There are many applications of crystalline solids, some are:
- Diamond is the most decent example of crystalline solids and is widely used in making beautiful jewelry items
- Quartz is extensively used in manufacturing of watches and clocks
- Many crystalline solids are used as a raw material in many industries
Image 4: Structure of NaCl
Crystalline Solids are further classified into four categories on the basis of intermolecular interactions between molecules, they are:
- Molecular Solids
- Covalent or Network Solids
- Ionic Solids
- Metallic Solids
Image 5: Shape of Crystalline and Amorphous Solids
Molecular Solids
In molecular solids, the constituent particles are molecules. Molecular solids are generally insulators and are soft in nature. The density of molecular solids is quite low. Based on nature of molecules molecular solids are further classified into three forms:
- Non-polar Molecular Solids
- Polar Molecular Solids
- Hydrogen-bonded Molecular Solids
Covalent Solids
In covalent solids, the constituent atoms of molecules held together by strong covalent bonds. They form giant structures and are generally hard in nature. They are also poor conductors of electricity except graphite, which is a good conductor of electricity.
Ionic Solids
The constituent particles in ionic solids are cations and anions. There is strong electrostatic force of attraction between particles. Ionic solids are hard and brittle. They have very high melting point. Ionic solids are poor conductor of electricity in solid state, but in dissolved state, they act as good conductor of electricity. For Example, NaCl is a good conductor of electricity in dissolved state.
Metallic Solids
The constituent particles in metallic solids are metal atoms and valence electrons. Metallic Solids have high melting and boiling point. They are also good conductor of electricity due to presence of valence electrons. For Example: Copper, Gold etc.
Difference between Crystalline and Amorphous Solids
The difference between crystalline and amorphous solids can be laid out in the table below:
Characteristic | Crystalline Solids | Amorphous Solids |
Melting Point
|
Melt at fixed temperature
|
Melts steadily over range of temperatures
|
Arrangement of Constituent Particles
|
Regular
|
Irregular
|
Shape
|
Regular and Definite Shape
|
Irregular Shape in Nature
|
Cleavage
|
When cut, two smooth and plain pieces are obtained
|
When cut, two surfaces of irregular shape is obtained
|
Heat of Fusion
|
Definite
|
Indefinite
|
Anisotropy
|
Anisotropic
|
Isotropic
|
Nature
|
True Solids
|
Pseudo Solids
|






No comments:
Post a Comment